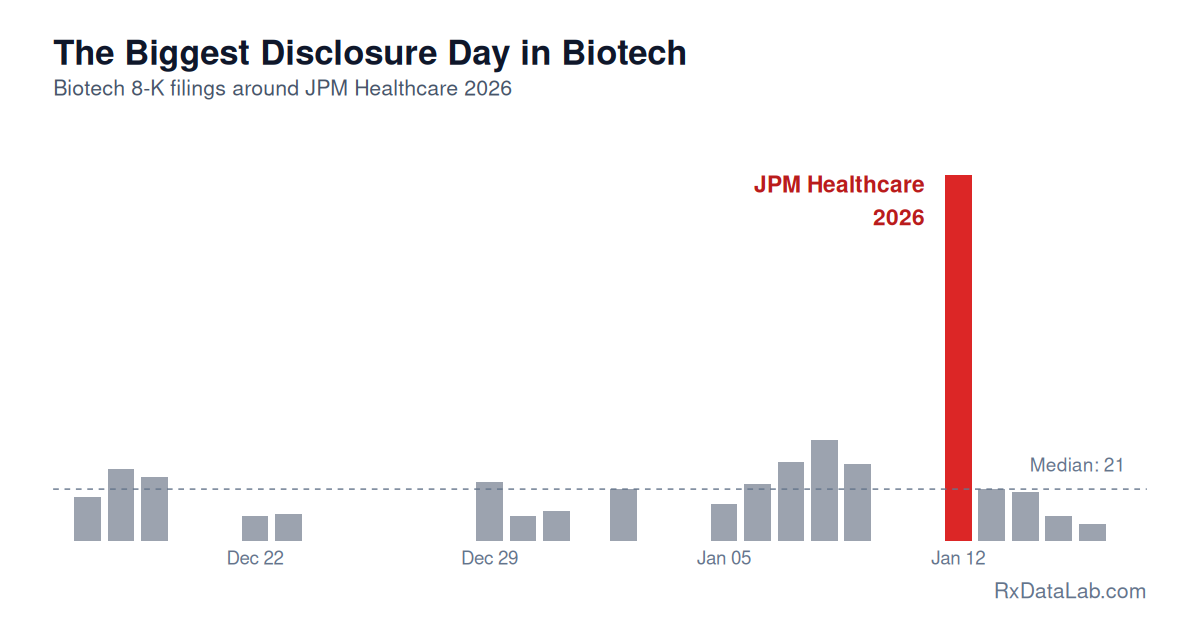
The Biggest Disclosure Day in Biotech
JPM Healthcare opening day triggers more biotech 8-K filings than any other day of the year. Analysis of SEC filing patterns shows 23% of public biotechs tracked by RxDataLab disclose on a single day.
My research ranges from quick analyses of market movements and companies to comprehensive deep dives on therapeutic areas and companies. All pieces are grounded in data and designed to cut through industry noise. I also publish educational guides on how I perform my research and publish general news and opinions in the blog.
In-depth, data-driven analysis on biotech companies and markets.
Technical content, news, and opinions on the biotech industry.
Tutorials on biotech industry research methods and details abdout data sources and regulatory filings.

JPM Healthcare opening day triggers more biotech 8-K filings than any other day of the year. Analysis of SEC filing patterns shows 23% of public biotechs tracked by RxDataLab disclose on a single day.
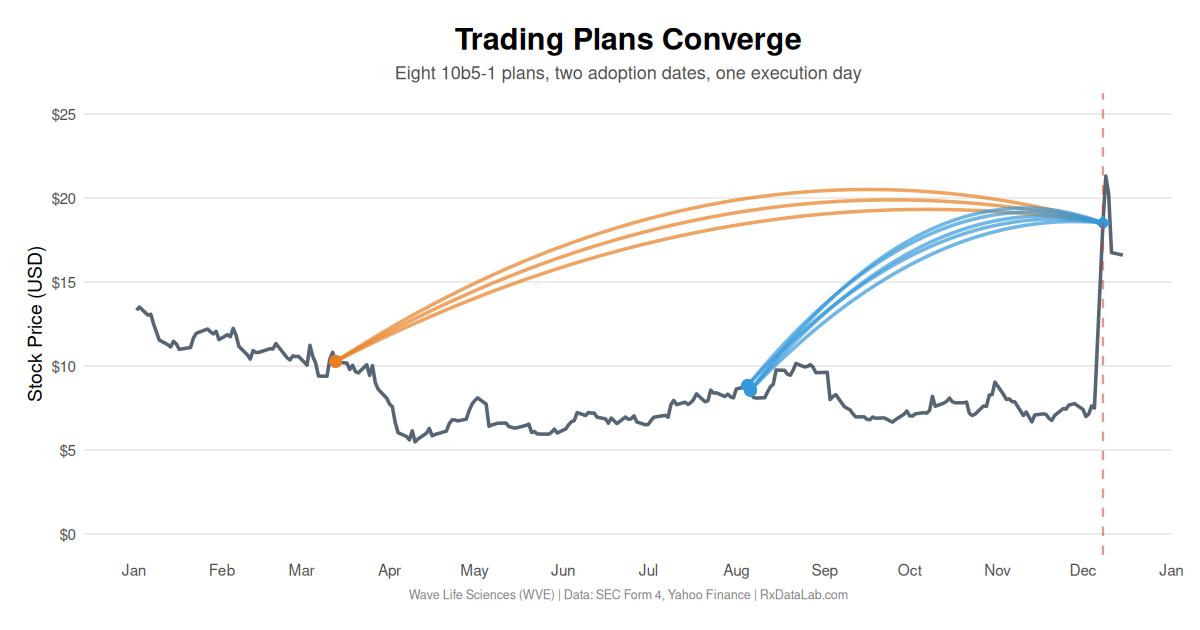
Eight Wave Life Sciences executives adopted 10b5-1 trading plans in two clusters months apart, and all eight plans executed on the day of a clinical trial announcement.
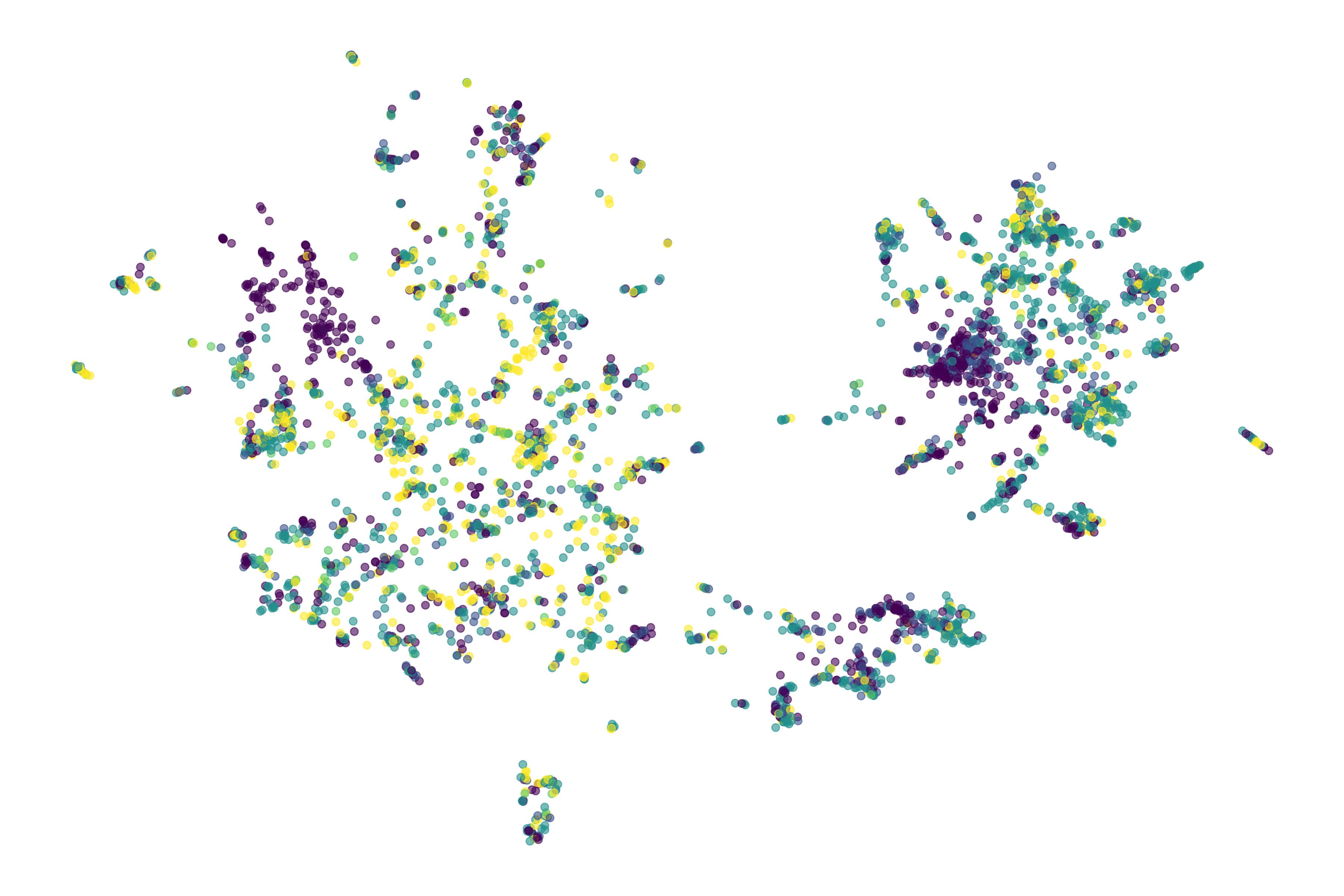
How we use embeddings of clinical trial features to visualize and analyze the competitive biotech landscape.
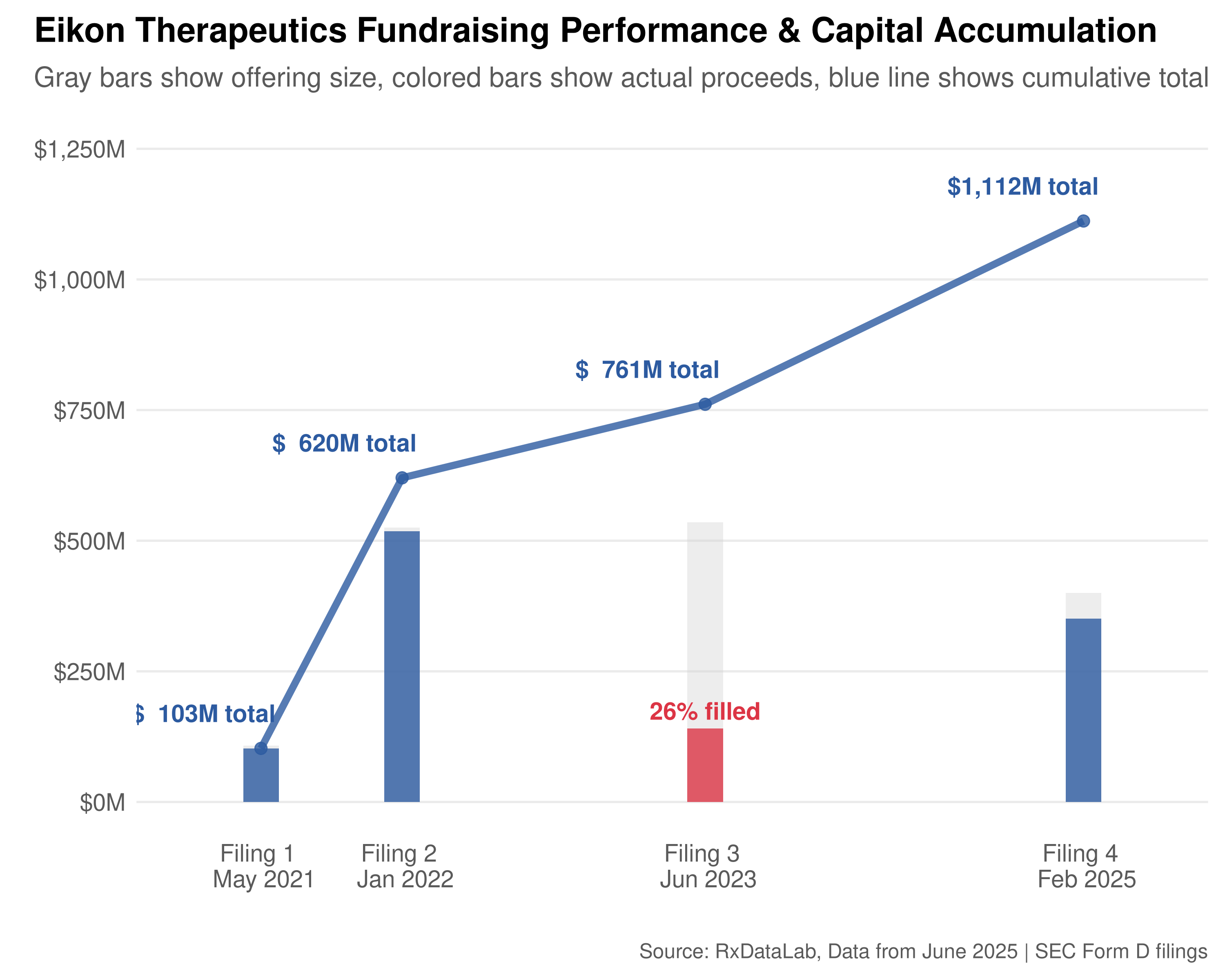
Eikon Therapeutics has raised over $1B and claims to have a revolutionary drug discovery platform, but its clinical pipeline remains dominated by acquired oncology assets. This report analyzes Eikon’s funding, trials, and patent strategy to assess whether its platform is delivering.
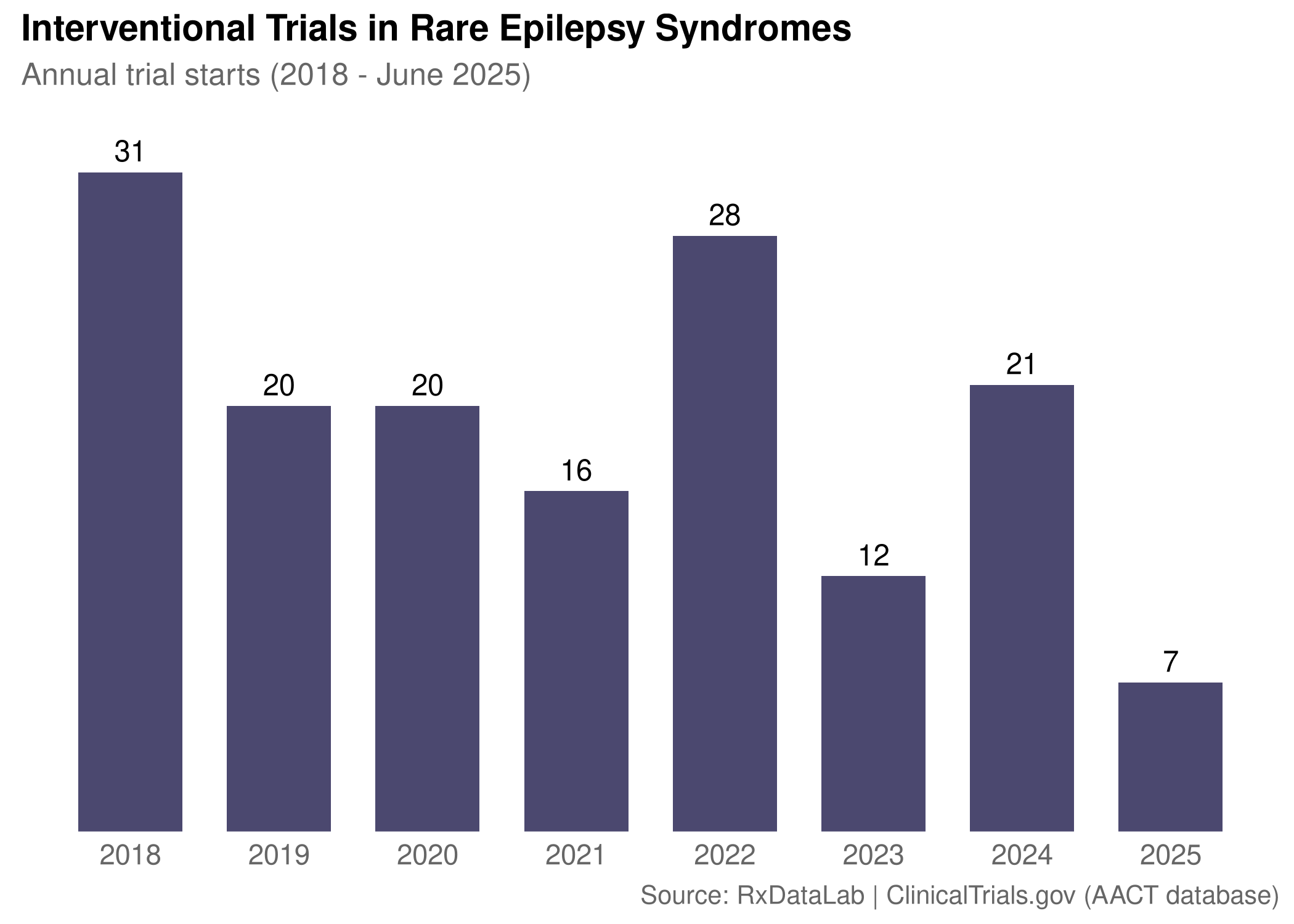
After Lundbeck’s $2B acquisition of Longboard, investors scrambled for serotonin analogs. Here’s why Bright Minds was the target and what it says about the future of rare epilepsy drug development.

Visualizing the rise in Investigational New Drug (IND) receipts and active INDs during 2019-2020. With no major regulatory changes, the spike appears linked to the COVID-19 pandemic, raising questions about how it shaped drug development.

81% of drugs approved in 2000 have generic equivalents in 2024. We will dive into how we used the Orange Book to answer this question, and how we approach these problems.

The FTC recently challenged over 100 Orange Book patent listings for drugs including blockbuster Ozempic. In fact, up to 89% of claimed patents were challenged for certain drugs. Here we dive into what was challenged, whether it matters, and what happens now.

Glucagon-Like Peptide-1 (GLP-1), Semaglutide, and other GLP-1 targeting drugs are involved in many clinical trials as of July 2024. Here, we cover some of the conditions with active clinical trials that may not be getting as much attention as the weight loss and diabetes cases.