RxDataLab
Nicholas George, Principal Consultant
Biotech Company Deep-Dive: Platform Claims vs Clinical Reality
Eikon Therapeutics Case Study
Analysis of Eikon Therapeutics' $1B+ fundraising, clinical pipeline, and platform claims using SEC filings, clinical trial data, and patent research.
Executive Summary
Key Findings
- Identified $1B+ fundraising through SEC Form D analysis
- Discovered two quietly terminated clinical trials in 2023
- Mapped pipeline showing 75% acquired vs developed assets
Business Impact
- Revealed gap between marketing claims and clinical reality
- Provided investors clear timeline for platform validation
- Identified key risk factors in current pipeline strategy
Technical Stack
- Python for SEC data scraping and XML parsing
- R (tidyverse) for data analysis and ggplot2 for visualization
- PostgreSQL for clinical trial data hosting
- Hugo/Markdown for automated report generation
Research Process
Multi-source validation approach: Cross-referenced SEC filings with third-party databases, tracked clinical trial status changes over time, and analyzed patent filing patterns to distinguish marketing claims from technical reality.
Fundraising Analysis

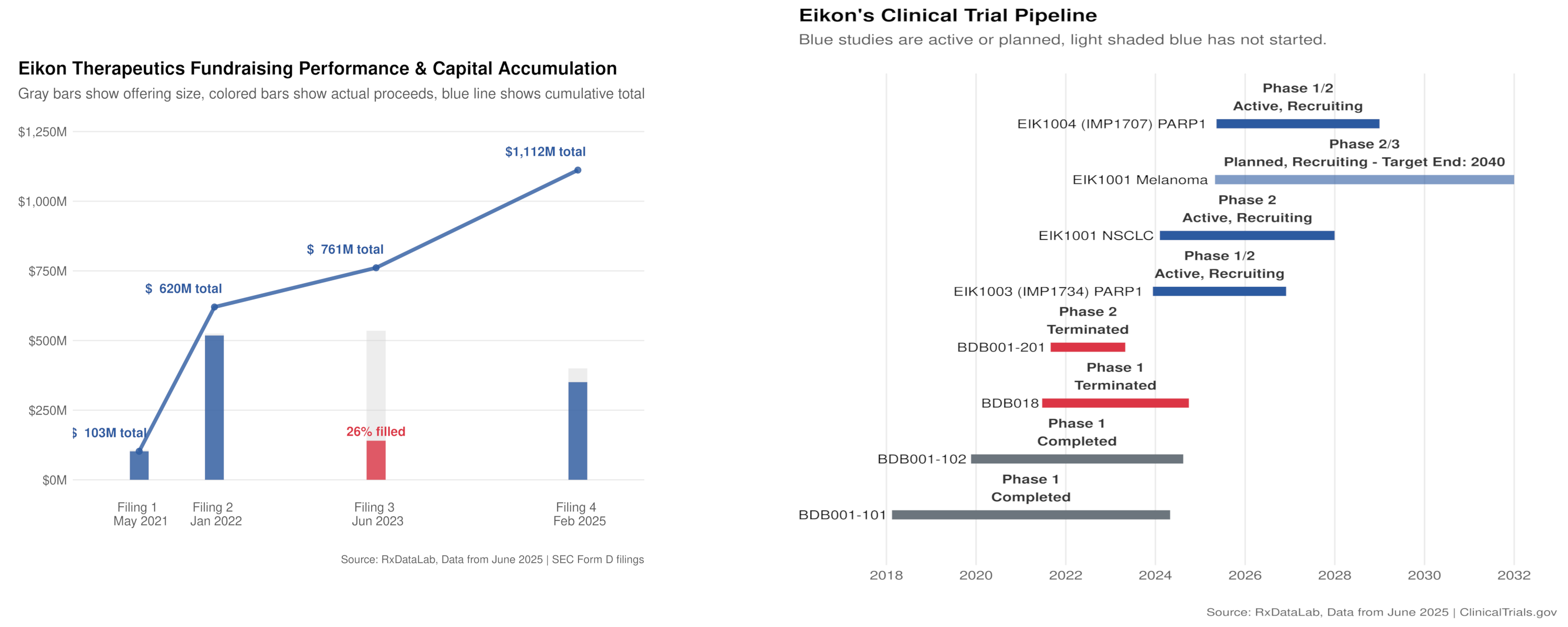
Eikon Therapeutics fundraising history based on SEC Form D filings
The fundraising pattern shows strong initial investor confidence followed by a significant struggle in 2023, raising only 26% of target. The recent $350M round suggests renewed confidence, likely tied to pipeline reset.
Clinical Trial Pipeline Analysis

Eikon's active and planned trials, showing reliance on acquired assets
Current pipeline is dominated by externally sourced compounds, with only EIK1005 (WRN inhibitor) representing potential platform-derived discovery. This suggests the revolutionary platform is still in development phase.
Methodology & Technical Approach
Data Sources
- SEC Form D filings for fundraising analysis
- ClinicalTrials.gov database for trial outcomes
- USPTO patent filings for platform development
- Company press releases and investor materials
Deliverables
- Executive summary with investment risk assessment
- Interactive funding timeline with investor participation analysis
- Clinical pipeline risk matrix with acquisition vs development breakdown
- Platform validation roadmap with key technical milestones
Ready for Similar Analysis?
This case study demonstrates systematic research methodology that can be applied to any biotech company or therapeutic area.
Contact: [email protected] | Portfolio: RxDataLab.com