RxDataLab
Nicholas George, Principal Consultant
FDA Orange Book Patent Exclusivity Database
Automated pipeline processing FDA Orange Book data to track pharmaceutical patent expirations and generic competition timelines
Live Dashboard: https://rxdatalab.com/data-products/exclusivity-dashboard/
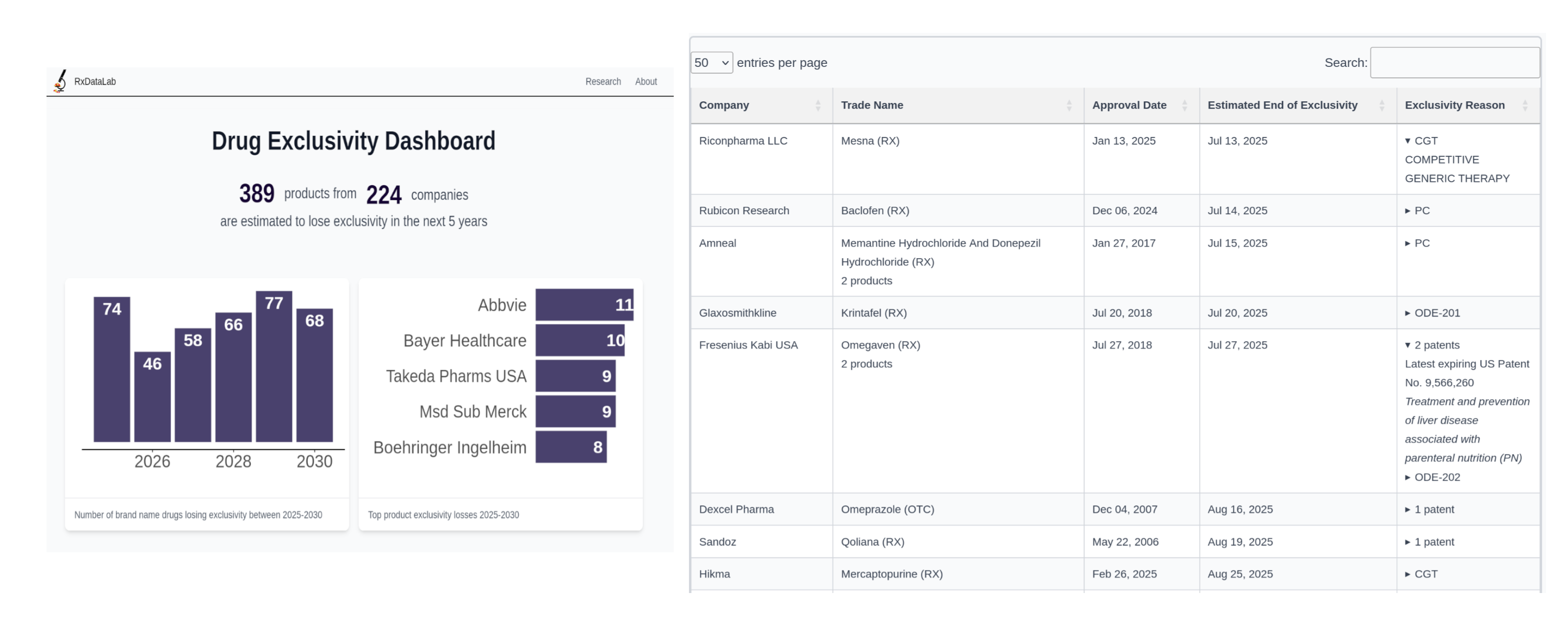
Interactive dashboard enabling search and analysis of pharmaceutical exclusivity data
Project Overview
Built comprehensive and interactive database tracking when brand-name drugs lose patent and FD&C Act exclusivity protection and become eligible for generic competition. Harmonized FDA Orange Book exclusivity data with USPTO patent information to provide complete pharmaceutical intelligence.
Key Features
- Searchable database of drug exclusivity records
- Automated regularly with data from FDA and USPTO
- Exclusivity expiration predictions using patent data and FDA exclusivity
- Generic competition timeline analysis
Technical Implementation
Data Sources
- FDA Orange Book exclusivity database
- USPTO patent filing records
- Regulatory guidance documents
Technical Stack
Python
SQLite
R
REST APIs
Data Processing Pipeline
Automated Pipeline: Downloads FDA Orange Book data monthly, harmonizes patent numbers with USPTO records, calculates exclusivity periods, and updates live database. Handles complex regulatory exclusivity rules and overlapping patent protections.
Technical Challenges Solved
- Calculating exclusivity periods with overlapping protections
- Building reliable automated updates for regulatory data changes
- Creating user-friendly search interface for complex pharmaceutical data
- Presenting data in an informative and user-friendly way
Impact & Results
- Enables pharmaceutical companies to identify generic opportunities
- Provides investors with patent cliff analysis for portfolio decisions
- Supports researchers tracking drug accessibility timelines