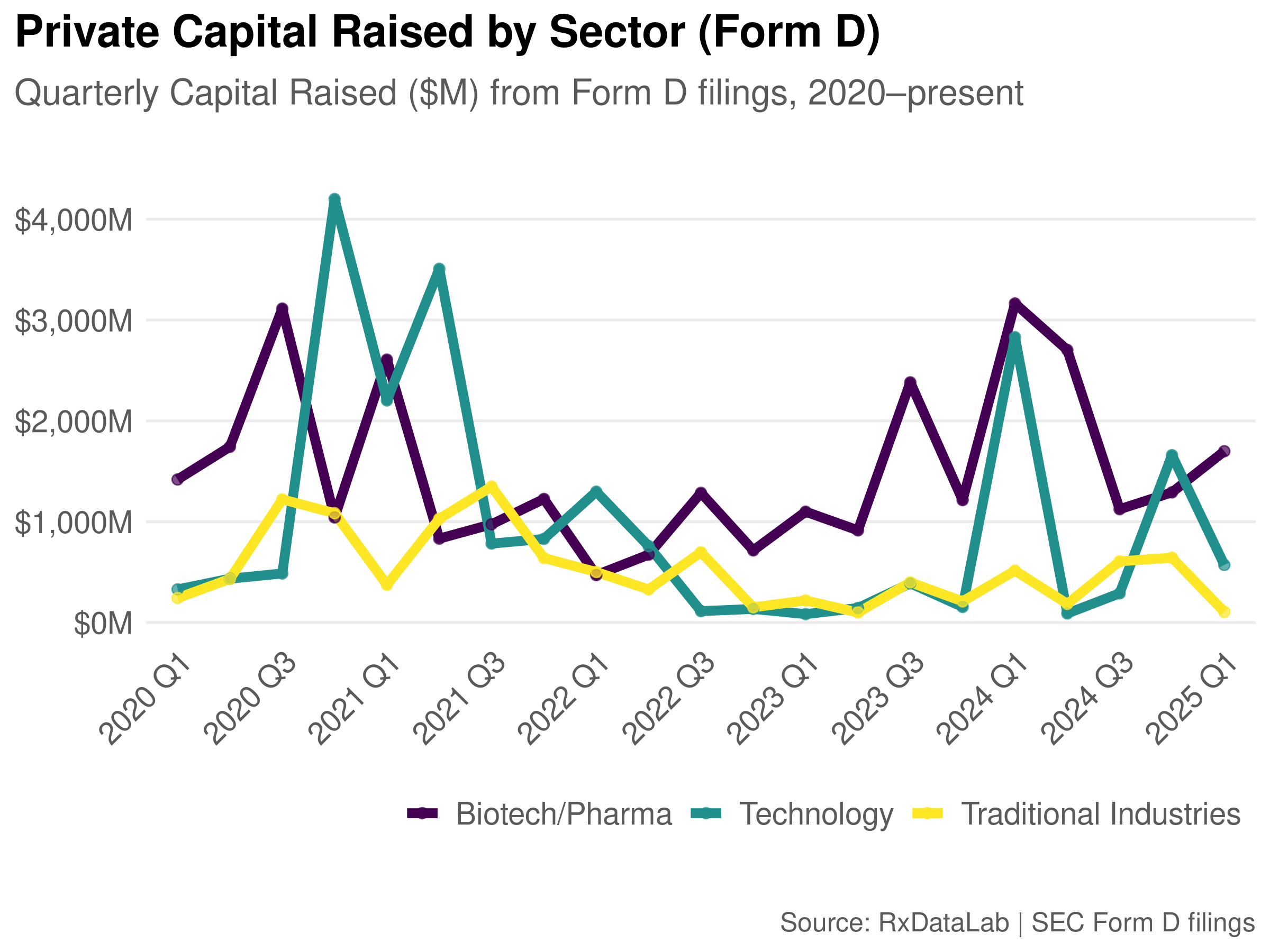
Building a Form D Database for Biotech Investment Tracking
Learn how to parse SEC Form D filings into a private capital database for biotech and venture research.
Clear explanations of the regulatory frameworks, processes, and fundamentals that drive the biotech industry.
Topics include FDA approval pathways, clinical trial design, regulatory compliance, and the business mechanics of biotech companies. We also publish original research on companies, technologies, and platforms, and we publish general news and opinions on our blog
In-depth, data-driven analysis on biotech companies and markets.
Technical content, news, and opinions on the biotech industry.
Tutorials on biotech industry research methods and details abdout data sources and regulatory filings.

Learn how to parse SEC Form D filings into a private capital database for biotech and venture research.
The drug development process consists of 5 distinct steps, and there are data sources available to learn more about each step. Here I will cover some of the most important for our work.

The Orange Book, formally known as "Approved Drug Products with Therapeutic Equivalence Evaluations", is a publication that provides consolidated intellectual property, exclusivity information, and therapeutic equivalents for FDA approved brand name drugs.
Understand the various types of exclusivity and intellectual property protections surround pharmaceutical products in the United States